
Hydrogen is being evaluated as a tool to decarbonize the energy system, because it emits no greenhouse gases at its point of use: when used to produce energy in fuel cells, hydrogen only produces water. If hydrogen is burned for energy instead, it produces water vapor and air pollutants, mainly nitrogen oxides.
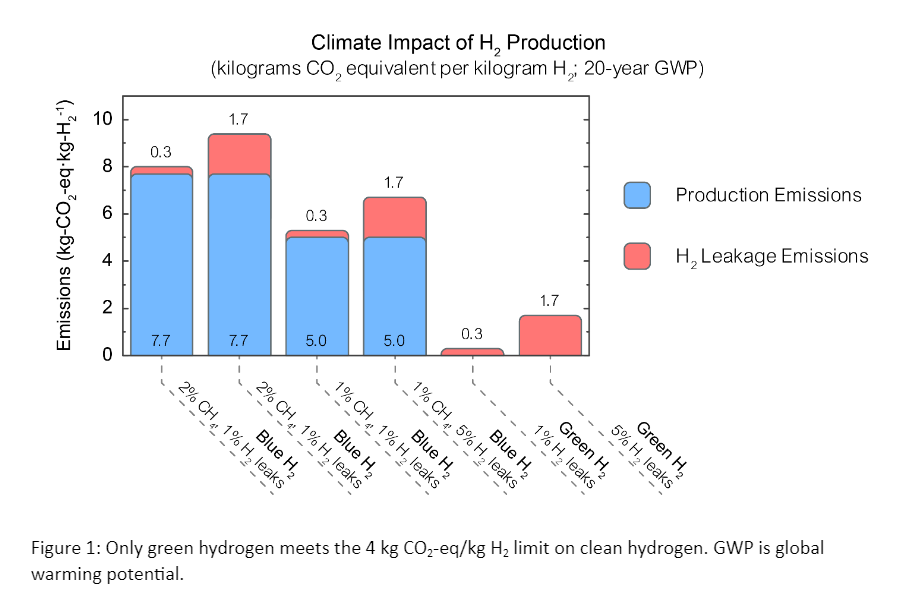
Greenhouse gases are emitted throughout the lifecycle of hydrogen production, starting from feedstock extraction through delivery to the production site. The Department of Energy (DOE) has published an aspirational standard for lifecycle greenhouse gas emissions of 4 kg CO2-eq from well-to gate. This includes CO2 sequestration but does not include any other activities at the site of production or post-production steps like liquefaction, compression, etc. Hydrogen hubs are not required to meet this standard.
Additionally, the standard does not include the impact of hydrogen leaks and evaluates methane (the main constituent of natural gas) at its 100-year impact instead of the 20-year impact that is relevant for 2050 climate goals.

Hydrogen Production
For hydrogen to help decarbonize the energy system, its production must have low or zero greenhouse gas emissions. Figure 1 shows the greenhouse gas emissions associated with common methods of hydrogen production.
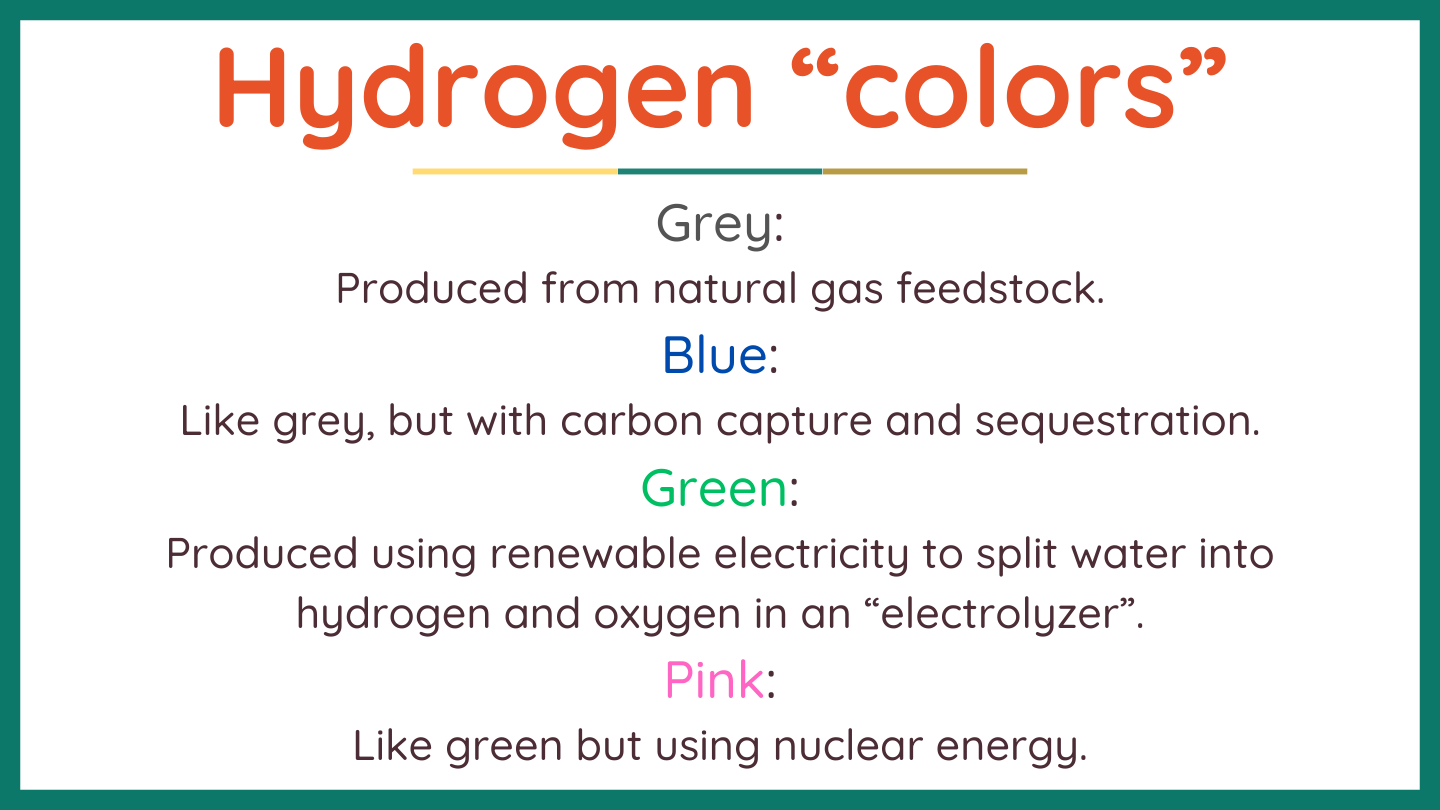
Hydrogen produced with different energy inputs and processes has been assigned “colors” for easy differentiation. The most important are shown here. Green and pink hydrogen have essentially zero greenhouse gas emissions at the site of production. However, if decarbonized electricity is diverted towards hydrogen production, the net consequences are generally very high emissions. Such diversion does not result in clean hydrogen and should not be incentivized. “Additionality” should be required (see box).

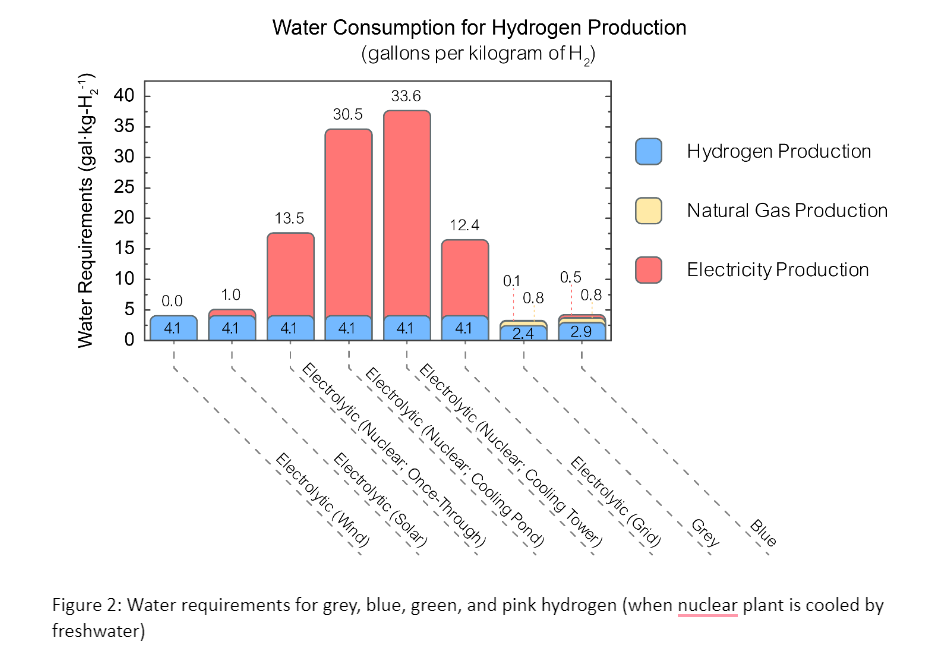
Water Requirements for Hydrogen
Hydrogen production is very water-intensive. Some or all of the hydrogen comes from its content in water (H2O): half in the case of production from natural gas, and all when electrolysis is used to split water. Using thermo-electric generation (e.g. nuclear or fossil-based power) to supply the energy increases water use dramatically, because of water requirements when cooling plants with freshwater (Figure 2; does not apply to seawater cooling). Such immense water use has serious implications for siting, for instance in water-stressed areas, notably the Westand Southwest. As fossil fuel plants retire, hydrogen production could become the second largest use of water (after agriculture) if the DOE’s “optimistic” estimate of hydrogen production is realized. There will be a reduction in water use and water pollution if green hydrogen displaces fossil-based hydrogen production, especially when displacing water-intensive fracked natural gas feedstocks. Local as well as global net water use and pollution impacts are important.
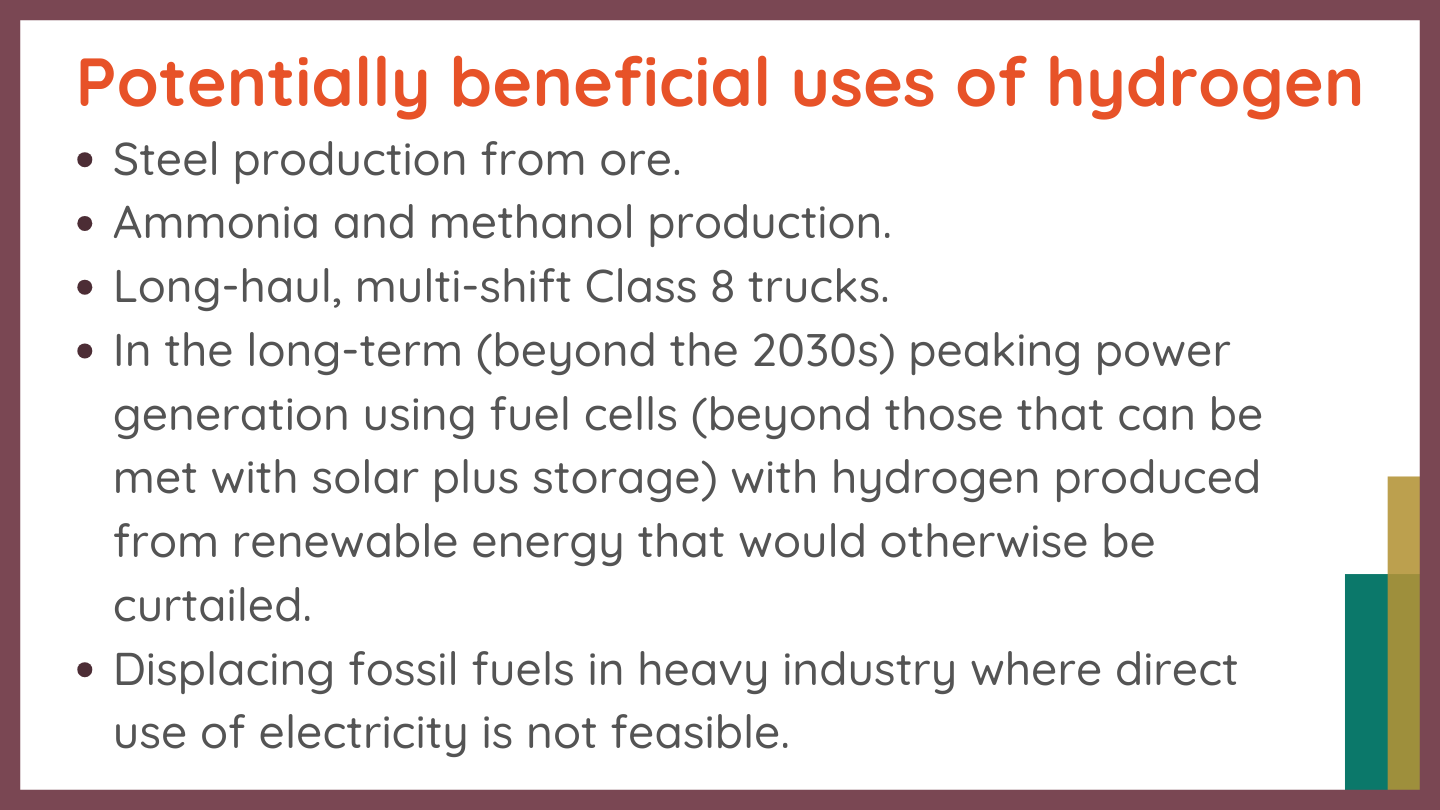
Hydrogen Uses
Many uses of (green) hydrogen would have marginal climate benefits or increase emissions. Even if emissions are reduced, hydrogen usage can have high opportunity costs: emissions could be reduced much more if renewable energy were used directly instead of making hydrogen with it first.
There are also areas where green hydrogen can reduce GHG emissions and provide other health and environmental benefits.

Environmental Justice
Hydrogen production, transportation, and use raise safety and environmental justice questions. For example, materials for catalysts used for electrolysis and for production from fossil fuels are often sourced from the Global South and/or Indigenous lands with serious environmental and health impacts; for example iridium and platinum come largely from South Africa and nickel from Indonesia. Large water requirements raise equity and water justice issues, including treaty rights of Indigenous Nations. Thus, strict protocols and their enforcement throughout the hydrogen supply chain are needed. Hydrogen is also flammable and explosive; safety rules need to be developed as hydrogen use expands, including in frontline communities.

